Microbiology – Metabolism:
Fueling Cell Growth
Chapter 8: Microbial metabolism
I. Principles of Metabolism
A. ____________________________ – the sum total
of chemical
reactions
used for biosynthetic & energy harvesting processes.
1. ____________________________ encompasses those
processes that generate
energy.
2. ____________________________ includes the processes that
utilize energy to
synthesize and assemble the building blocks of a
cell.
a. cell walls, membranes, ribosomes, nucleic
acids, &
surface
structures
B. Harvesting Energy
1. Energy
is the ability to do ____________________________.
a. ________________________ energy – energy of motion
b. ________________________ energy – stored energy
c. The
first law of thermodynamics states the energy in a
closed
system can never be created or destroyed; it can be
changed from
one form to another.
d. the second law of thermodynamics
states that
_______________________________________________.
1) Entropy is a tendency toward disorder &
randomness (the
production of heat)
2. Types of Microbes
a.
Phototrophs harvest the energy of sunlight, using it to
power
the synthesis of organic compounds.
b. Chemoorganotrophs get energy by
degrading organic
compounds,
releasing the potential energy of their chemical
bonds.
1) ____________________________ is the amount
of energy that can be gained by
breaking the bonds of
a chemical.
a) ____________________________
reactions
release energy;
b)
____________________________
reactions
utilize energy.
C. Components of Metabolic Pathways
1. A metabolic pathway can be linear,
branched, or cyclical,
generating a sequential
series of __________________________
and, ultimately, an ____________________________.
a. Cells must be able to carry out chemical
reactions
quickly. How would you speed up a reaction? (Imagine
dissolving
sugar in water.)
2. A specific ____________________________ facilitates each
step of a metabolic
pathway by lowering the activation energy of a
reaction that converts a
substrate into a product.
a.
Enzyme
names typically end in ____________________.
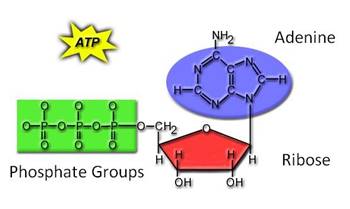
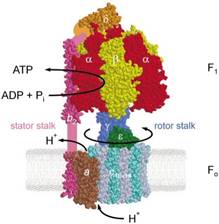
3. ____________________________ is the energy currency of the
cell.
a. _____________________________________________
uses
the chemical energy released in an exergonic reaction
to
add Pi to ADP.
b. ______________________________________________
harvests
the energy of proton motive force to do the same
thing.
1)
_______________________________________
is generated as electrons are passed
along the
electron transport chain
2)
Often, ____________________________
acts as
the terminal electron acceptor
|
Did You Know ATP Recycling in Your
Body Is AMAZING? |
|
The total quantity of ATP in the human
body is about 0.1 mole. The majority of ATP is not usually synthesized from scratch, but is recycled from
ADP + P used by the body in metabolic reactions. Thus, at any given time, the total amount
of ATP + ADP remains fairly constant. The energy used by human cells requires
the hydrolysis of 100 to 150 moles of ATP daily which is around 50 to
75 kg. Typically, a human will use up their body weight of ATP over the
course of the day. This means that
each ATP molecule is recycled 1,000 to 1,500 times during a single day. Because ATP cannot be stored, cells use it
for energy almost immediately after its synthesis. If a car was as efficient at converting
fuel to motion as your cells are at converting the energy stored in sugar
into energy stored in ATP, you could drive 100,000 miles on a single tank of
gas! |
4. Oxidation-reduction reactions
Helpful Acronym: Oxidation Is Losing Electrons, Reduction
Is Gaining Electrons: OIL RIG
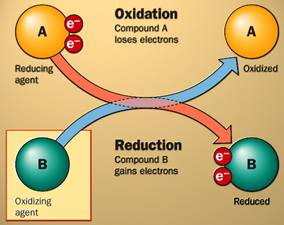
Oxidation – the loss of electrons &/or hydrogen (the loss of energy)
Reduction – the gain of electrons &/or hydrogen (the
gain of E)
a. When a substance gives up an electron
(energy), we say
that it has
been ____________________________.
1) Imagine burning wood. The wood is becoming
oxidized
& being converted to ash. The ash
doesn’t
have
the potential energy that the wood had.
We can
see
the energy escaping as heat and light (fire).
b. When a substance accepts an electron
(energy), we say
that is has
been ____________________________.
c. When electrons are transferred from one
compound to
another,
they often travel paired to a proton.
This is an
electron-proton
pair, or ____________________________.
1) When substances lose hydrogen, they are
oxidized,
when they gain hydrogen, they are reduced.
d. When electrons are removed from an energy
source, or
electron
donor, during catabolism, they are temporarily
transferred
to a specific molecule that acts as an
_______________________________________________.
1) The electron carrier becomes reduced
5. Electron carriers
a. Three freely diffusible electron carriers
are…
1) ___________ (nicotinamide adenine dinucleotide)
2) _________________ (flavin adenine dinucleotide)
3) ________________________ (NAD phosphate)
b. Their reduced forms
function as ____________________
(their bonds hold a form of
usable energy).
1) NADH & FADH2 are used to
provide electrons for
the
generation of the proton motive force
a) This drives the synthesis of ATP in the
process
of oxidative phosphorylation
2) NADPH is used in biosynthetic reaction when a
reduction is required
6. _________________________________________________
are
metabolic intermediates that link anabolic & catabolic pathways.
a. They are produced in catabolic pathways &
can be further
oxidized
to generate energy.
b. They can used in anabolic pathways, serving
as the
building
blocks to make the subunits of macromolecules
II. ____________________________ – proteins
that function as biological
catalysts;
they facilitate the conversion of a substrate into a product; they are
neither
consumed nor permanently changed during a reaction.
A. Mechanism and Consequence of Enzyme Action
1. The
substrate binds to the active site or ___________________
to form a temporary
intermediate called an
____________________________.
a. The substrate is held w/in this complex in a
way that
lowers the
activation energy for a given reaction
b. The products are released, the enzyme is
unchanged
B. Allosteric regulation
1. Cells can fine-tune the activity of an
____________________________
by using an effector that binds
to
the allosteric site of the enzyme. This binding alters the relative
____________________________
of the enzyme for its substrate.
2. Provides the cell w/a means to modulate the
pace of metabolic
processes,
turning off some pathways & activating others
3. e.g. ________________________________________ – the
product
of a pathway effectively modulates its own synthesis (as in
tryptophan
production)
C.
Cofactors and coenzymes
1. Enzymes sometimes act in conjunction with ________________
such
as coenzymes and trace elements.
2. ____________________________ (such as CoA) transfer
substances
(such as the acetyl group) from one compound to
another
3. Most coenzymes are synthesized from vitamins
4. If a coenzyme is missing, the function of all
the different
enzymes
whose activity requires that coenzyme will be impaired
D. Environmental Factors That Influence Enzyme
Activity
1. The growth of
any organism depends upon the proper
functioning of its enzymes
2. Enzymes have a
narrow range of environmental factors –
including temperature, pH, and salt concentration – at
which it
operates optimally
3. A 10˚C
rise in temperature ~doubles the speed of enzymatic
reactions, until optimal activity is reached
a. If
the temp. gets too high, the proteins become denatured
& no longer function
E.
Enzyme Inhibition
1. Competitive inhibition occurs when the
inhibitor competes with
the
normal substrate for the active binding site.
2. Non-competitive inhibition occurs when
the inhibitor and the
substrate
act at different sites on the enzyme.
a. Allosteric inhibition can be considered an
example
III. Scheme of Metabolism
in Aerobic Chemoorganotrophs
A. Chemoorganotrophs include most bacteria &
all eukaryotic organism
except plants
& algae
1. They obtain energy by oxidizing organic
compounds
2. Because they also obtain C from organic
compounds, they can
also
be chemoheterotrophs
B. The
___________________________________________________
together gradually oxidize ___________________________
completely to
carbon dioxide
1. the pathways release energy that can be
harvested to generate
ATP
& accumulate reducing power
2. The central metabolic pathways also form precursor
metabolites
3. The central metabolic pathways are…
a. ____________________________ = glycolytic pathway
=
Embden- Meyerhoff pathway
b. _____________________________________________
c. _____________________________________________
(TCA
cycle) = Krebs cycle = citric acid cycle
C.
____________________________
(glycos “sugar” lysis “dissolution”)
– the most common pathway that
initiates the breakdown of sugars
1.
2. Yield of Glycolysis
a. A small amount of energy – net yield of 2 ATP
1) It takes 2 ATP to complete glycolysis, and 4
ATP
are
generated
b. Some reducing power in the form of 2 NADH + 2
H+
c. 6 different precursor metabolites
1) 5 intermediates of glycolysis & the end
product,
pyruvate
2) The precursors can be funneled off for
biosynthesis,
but this reduces the amount of ATP &
reducing
power produced by glycolysis
D.
___________________________________________________
– also
converts glucose to pyruvate
yielding a small amount of energy
1. Its primary role is the production of
compounds used in
____________________________, including
a. Reducing power in the form of NADPH
b. 2 different precursor metabolites
E.
___________________________________________________
–
pyruvate must be converted into a
specific two- carbon fragment to enter
the Tricarboxylic Acid Cycle (TCA or
Krebs cycle)
1. Steps involved…
a. A carbon dioxide is removed from pyruvate (3
C)
1) This produces a __________________________
b. An oxidation occurs, reducing NAD+
to 2 NADH + 2 H+
c. The acetyl group is joined to a compound
called
____________________________
1) This forms acetyl-CoA (a two-carbon molecule)
2)
Note that for every molecule of glucose, 2
molecules
of acetyl-CoA are produced
2. Yield of Transition step
a. Reducing power in the form of 2 NADH + 2 H+
b. 1 important precursor metabolite,
____________________________
F.
____________________________
(or Krebs cycle) completes the
oxidation of glucose
1.
2. For every molecule of glucose, the TCA cycle
must “turn” twice,
once
for each of the pyruvate molecules.
3. Acetyl-CoA goes through a series of oxidation
steps to release
two
molecules of carbon dioxide.
4. Yield of the two “turns” of the TCA
a.
A small amount of energy in the form of 2 ATP
b. A GREAT deal of reducing power in the form of
6 NADH
+ 6 H+
and 2 FADH2
c. 3 different precursor metabolites
G.
____________________________
– uses accumulated reducing
power to generate ATP by oxidative phosphorylation
1. Electrons carried by ____________________________ and
____________________________ are transferred
to the electron
transport chain
a. Membrane-embedded carriers accept a proton (H+)
electron
pair (or ____________________________)
b. These carriers pass the pair to another
membrane-
embedded
carrier that only accepts __________________
c. The freed protons are then shuttled from
inside the
membrane to
outside the membrane
d. This generates a ____________________________
e. The resulting NAD+ and FAD+
are recycled to be used
again
f. In aerobic respiration, ____________________________
(an
inorganic molecule) is the terminal acceptor of these
electrons
1) ½ O2 + 2 e- + 2 H+
à ______________________
(a.k.a.
metabolic water)
2. ATP synthesis
|
|
a. _________________________
harvests the energy of the proton motive force to synthesize ATP b.
For each pair of electrons transferred to the electron transport chain
by NADH, __________________________
are produced c.
For each pair of electrons transferred by FADH2 ____________________________
are produced |
3. Yields of ATP from aerobic oxidative
phosphorylation
a. From glycolysis: 2 NADH à _________________
b. From transition step: 2 NADH à _____________
c. From TCA cycle:
6
NADH à_________
and 2 FADH2 à
__________
1) 18 + 4 = 22 gained ATP
H.
ATP Yield of Aerobic Respiration in Prokaryotes
1. From Substrate-Level Phosphorylation:
2 ATP (from glycolysis, the
breakdown of glucose to pyruvate)
+ 2 ATP (from TCA cycle, the
breakdown of pyruvate to carbon dioxide
4 ATP (total gain form
substrate-level phosphorylation
2. From Oxidative Phosphorylation (from the
reducing power of
NADH &
FADH2)
6 ATP (from the reducing power gained in
glycolysis)
+ 6 ATP (from the reducing power gained
in the transition step)
+ 22 ATP (from the reducing power
gained in the TCA cycle)
34 ATP (total gained from oxidative
phosphoylation)
3. TOTAL ATP gain = 4 + 34 = 38 ATP
IV. Scheme of Metabolism in Anaerobic
Chemoorganotrophs
A. Glycolysis and the
pentose phosphate pathways are used
____________________________ to oxidize glucose to
pyruvate.
B.
______________________________________________________
–
Unlike eukaryotes, some
prokaryotes can respire using an inorganic
molecule other than
molecular oxygen as a terminal electron acceptor.
1. Anaerobic
respiration generates less energy than aerobic
respiration, and alternative electron carriers are used
in the electron
transport chain.
C. ____________________________ – used by microbes that cannot
respire, either because a suitable inorganic terminal electron
acceptor is
not
available or because they lack an electron transport chain.
1. Results in only partial oxidation of glucose
& thus produces
relatively little ATP
a. In general the only
ATP-yielding reactions of
fermentations
are those of the glycolytic pathway; the other
steps
provide a mechanism for recycling NADH.
2. Microbes use pyruvate or a derivative of it
as a terminal electron
acceptor
a. This recycles the reduced electron carrier
NADH
3. Fermentation produces endproducts (acids,
alcohols, &/or
gases) that
still have a lot of potential energy when compared to the
end product
of respiration, carbon dioxide
a.
Some fermentation endproducts are commercially
valuable. ____________________________ is important in
the production of foods such as
cheese and yogurt.
____________________________ is used to make
alcoholic beverages and breads.
4. Because a given type of organism uses only
one pathway,
fermentation
endproducts can be used as identifying markers.