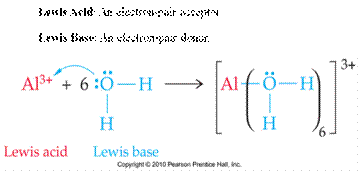
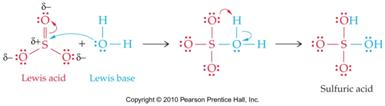
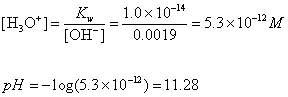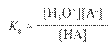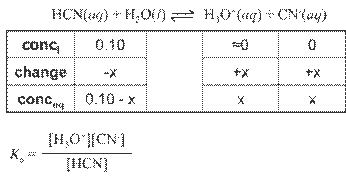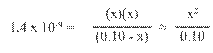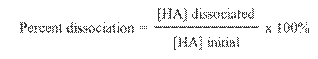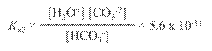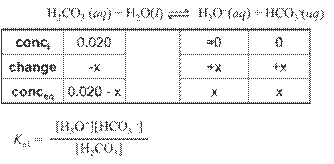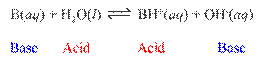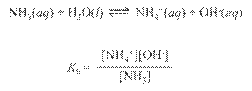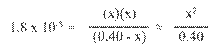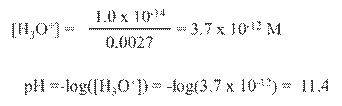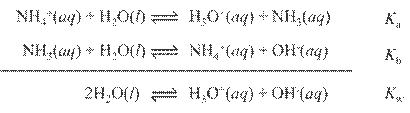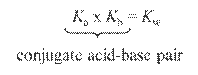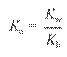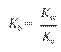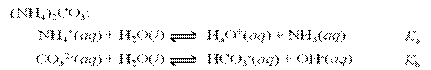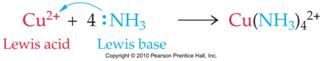Acids and Bases
return
Properties of Acids
Sour taste
Change the color of indicators
litmus - blue to red
bromcresol - green to yellow
phenolphthalein - rose to colorless
React with metals to liberate hydrogen gas
2HCl + Mg ® MgCl2
+ H2
Properties of Acids
React with basic metal oxides & hydroxides to form a salt
& H2O
2HBr + CuO(s) ® CuBr2 + 2H2O
React with salts of weaker or volatile acids to give a new
salt and a new acid
2HClO4 + FeS ® H2S + Fe(ClO4)2
+ 2H2O
Properties of Bases
Bitter taste
Change the color of indicators
litmus - red to blue
bromcresol - yellow to green
phenolphthalein - colorless to rose
Neutralize acids
Acid-Base Concepts: The
Brønsted-Lowry Theory
Arrhenius Acid: A substance that dissociates in
water to produce hydrogen ions, H+.
Arrhenius Base: A substance that dissociates in
water to produce hydroxide ions, OH-.
![]()
Bronsted-Lowrey Acid: A substance that can transfer hydrogen ions,
H+. A
proton donor.
Bronsted-Lowrey Base: A substance that can accept hydrogen ions, H+. A proton acceptor.
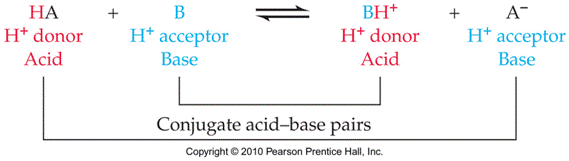
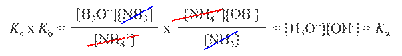
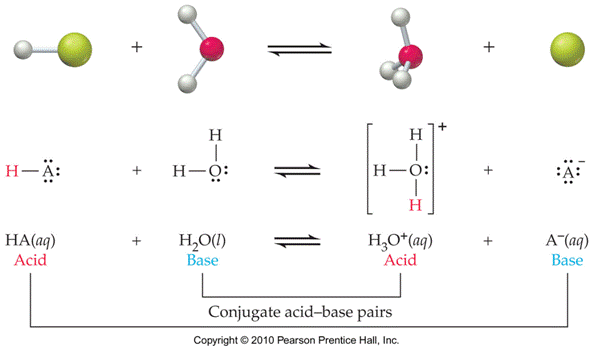
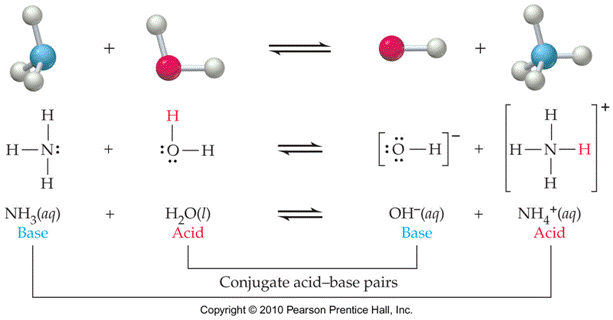
Conjugate
Acid-Base Pairs: Chemical species whose
formulas differ only by one hydrogen ion, H+.
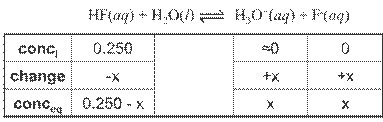
Acid Strength
Strong acid: near 100%
ionization
Weak acid: 10% or less
ionization; Only partially dissociated in water thus
being a weak electrolyte.
Base
Strength
Strong
acid: near 100% ionization
Weak acid: 10% or less
ionization; Only partially dissociated in water thus
being a weak electrolyte.
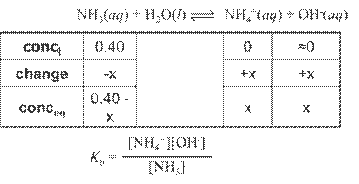
Hydrated Protons and Hydronium Ions
HA(aq)
↔ H+ (aq) + A- (aq) Due to high reactivity of the hydrogen ion,
it is
actually hydrated by one or more water molecules.
(For our
purposes, H+ is equivalent to H3O+.)
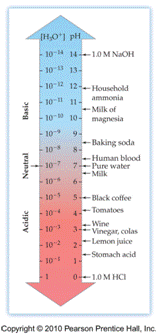
Dissociation
of Water
Ion-Product Constant for Water: Kw =
[H3O+][OH-]
At 25oC: [H3O+]
= [OH-] = 1.0 x 10-7 M
Therefore: Kw
= (1.0 x 10-7)(1.0 x 10-7) = 1.0
x 10-14
![]()
![]()